New trends in digital tools
for innovative clinical trials
Regarding decentralized clinical trials (DCTs) there are more and more new technological tools and innovative strategies that are used to highlight the importance of the participant as the primary core of clinical research. In that sense, reducing the burden of patients is of special interest and that is why research centers, sponsors, and contracted research organizations (CROs) must know what they are and thus promote their use in clinical trials.
Updated on June 14, 2023

Blog >
New trends in digital tools for innovative clinical trials
Some of these initiatives are devices specially designed to facilitate the process of inclusion, adherence, and data capture in such a way as to reduce the number of visits to the center, a situation that is very valuable in studies that include patients with limited mobility or those in which for different reasons (work, distance, and other responsibilities) make it difficult for them to fulfill all visits to the center. Other of these initiatives are new strategies that require interoperability with stakeholders to ensure that each activity of the study adapts in the best possible way to each participant and that he completes all the activities of the studies.
Now, these tools will only be "medical devices" if we do not use them in the right way, by this I mean that beyond a state-of-the-art equipment what really matters is that they are used in the right participant, at the right time, and in the right study, thinking that each strategy must be perfectly engaged with another one so that the data arrive complete and of quality at the time of analyzing them.
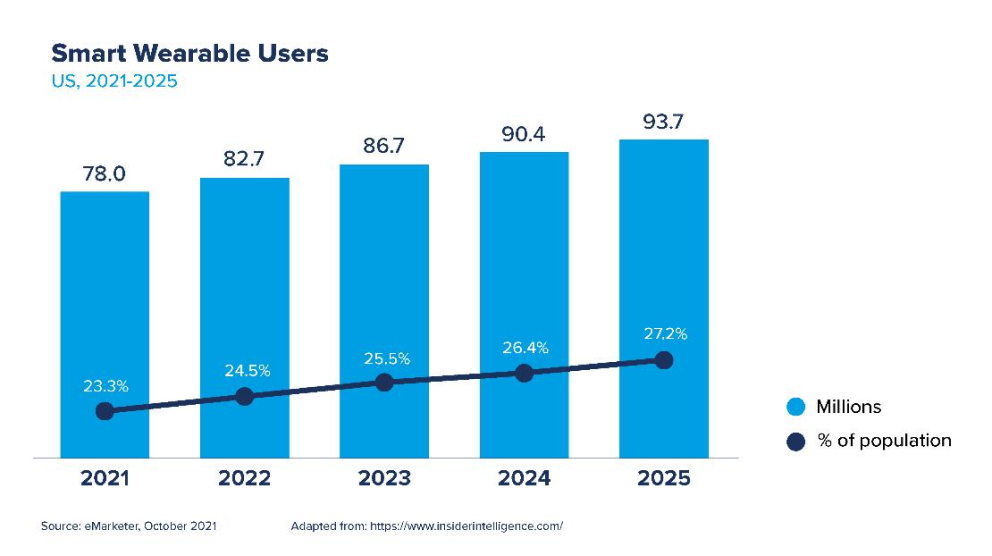
Wearable technologies are electronic medical devices that those who use them can easily wear or carry, their main objective is to capture biometric data and track their health status that the consumer can review quickly and that can be sent to large databases. According to a publication by Insider Intelligence, there is a growing trend to use these devices in the North American population, and it is estimated that by 2025 around 27.2% of its population will use them.
There are more than 40 different portable devices that are currently being used for real-time information capture. Some examples of these are:
Portable fitness trackers.
They were among the first initiatives to monitor heart rate, the number of steps taken, and physical activity, with adjustments of goals and recommendations.
Smart watches.
Which have presented an impressive evolution becoming important medical devices for self-care and prevention. For example, the Apple Watch Series 8 not only contains heart rate notifications and an electrocardiogram application but also temperature sensors to record women's health including ovulation, crash detector with which severe collisions can be detected that will trigger automatic call to emergency services.
Portable electrocardiographic monitors.
Clinically validated devices such as Mov ECG, allow to take in real time an electrocardiographic trace once symptoms such as palpitations are present, each event can immediately be sent to the doctor and stored on the clock until the next synchronization.
Portable blood pressure monitors.
Omrom is a pioneer in this type of device not only offers digital blood pressure monitors that send reports to an application where health care provider can review them in real time, but also offers watches that directly take blood pressure with the same advantages of immediate reporting and generation of alerts.
Biosensors.
They usually are introduced as patches that attach to the skin and have different uses from vital signs monitoring to enzyme-based biosensors, tissues, immunosensors, and DNA biosensors. An interesting proposal of these biosensors are the ingestible ones, all supported by biomedical engineering.
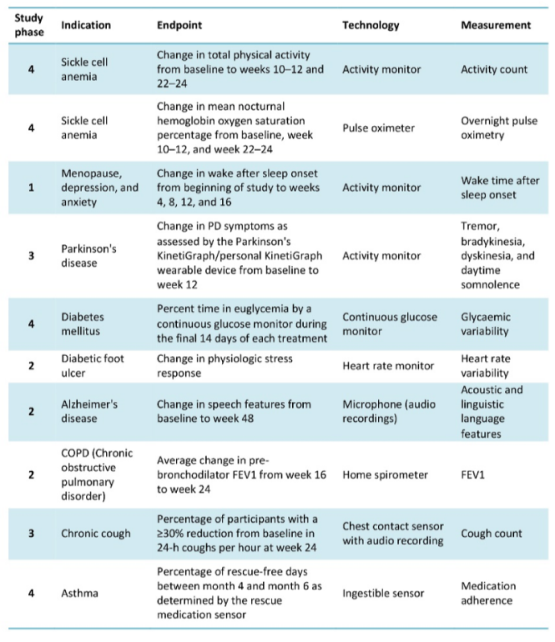
In a publication by Landers et al. of the Digital Biomarkers journal (2021), some examples of clinical studies that use electronic devices to meet the endpoints of the study are exposed, one of them even uses ingestible biosensors for an asthma study:
Digital strategies of clinical trials
Just as wearable devices are essential in this new interest in facilitating data capture, new technological strategies are also being implemented for the rigorous operational processes that occur in clinical trials. In this sense and driven by the COVID-19 pandemic, the Food and Drug Administration (FDA) published the guide: Digital Health Technologies for Remote Data Acquisition in Clinical Investigations in which recommendations are given for sponsors, researchers, and other actors on the use of digital health technologies to acquire data remotely from clinical research participants they evaluate medical products.
That guide not only talks about hardware related to medical devices but also about all software applications through platforms that facilitate the processes of clinical studies. These platforms are increasingly implemented in clinical trials and their acronyms are becoming more familiar:
Electronic clinical outcomes assessments (eCOAs) that include electronic instruments of patient-reported outcomes (ePRO) and electronic performance outcome instruments (ePerfO).
Other technological strategies that are already being widely adopted are: electronic informed consents (e-consent) which not only reduce times and costs but also open the opportunity to include more diverse populations in the studies since it offers the opportunity to enter subjects who would otherwise never participate, a situation that occurs in very sick patients, with mobility limitations or access difficulties. So that this process does not lose the rigor with which it must be applied, regulatory entities, sponsors, research centers, and patients must be aligned and based on updated guidelines in which the door is opened to these new trends.
Direct to Patient (DtP) solutions is a strategy published in the Clinical Trials Applied journal, where it is proposed that a large part of the study activities reach the participant directly, reducing the number of visits to the research center. For example, medicines arrive directly from the provider to the participant's home or also through "third parties" who are responsible for that shipment from the research center or central pharmacy. While expenses can be increased, these will be offset by greater adherence of subjects, less waste of research product, and decreased time to meet endpoints.
The future in clinical trials.
Surely what we are seeing as innovative strategies to 2022 in 5 years will no longer be and by then we will talk about how to implement other methodologies that today we see far away but that are already beginning to attract attention in clinical trials. Terms like artificial intelligence, virtual reality, machine learning and metaverse will be more common and will make us think, again, in a different way.
Along with the advancement of these technologies, doubts arise about the way these solutions should be implemented in such a way that we can trust them and that the data they provide is really true. In this sense there is also a lot of controversy especially because the validation processes that these initiatives have are questioned, this is something that traditional clinical trials have very well controlled given the rigor with which these studies are executed, so although we are in a trend of new devices and solutions you should never lose sight of the fact that each device, strategy or methodology must be consistent and even better that it has gone through all the validation and information security processes before implementing its widespread use.
Likewise, these proposals will be a challenge for the participants who will also have to learn to use them, a situation that could be a reason not to participate in the studies. The FDA is clear in recommending that informed consent forms include all information related to the use of platforms and other devices that may affect subjects' decision to participate in studies.
It is our duty as clinical research actors to promote all the positive aspects involved in the use of automated strategies and to take advantage of these tools to improve recruitment and adherence rates. This implies involving patients actively in the studies through recognition of compliance with goals in real time, the possibility of seeing their progress in the study, feedback, and timelyn response of the data they provide, making them feel that they are part of a group that shares the same ideas and other strategies that we can use thanks to technology.